
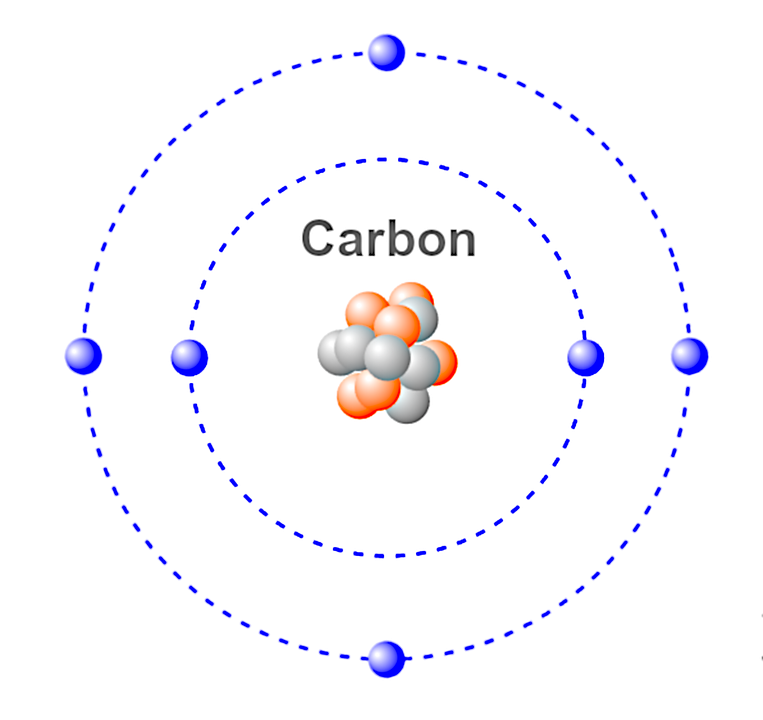
However, it was not until the 18th century that chemists looked more closely at the case of carbon. In ancient Asia, carbon was already present in the form of diamonds. History of Carbonįrom ancient times, the Romans began to make carbon with charcoal by techniques of carbonizing wood underground. Carbon atoms in compounds exhibit valence IV, III, II, I. The Carbon valence characterizes the ability of the C atom to form chemical bonds. The Ionization energy of Carbon, E o = 1086 kJ/mol. Quantum numbers are determined by the last electron in the configuration for Carbon atoms, these quantum numbers have the value N = 2, L = 1, M l = 0, M s = ½. The carbon atom and N +1, O +2 have the same electronic configuration.Ĭarbon atoms in compounds have oxidation states 4, 3, 2, 1, -1, -2, -4. You can also write carbon electron configuration in short form as- 2s 2 2p 2Ĭarbon has 6 electrons, you can fill the electrons as per below: The electronic configuration of carbon in the state of minimum energy or base is 1s 2 2s 2 2p 2. Its atomic number (Z) is 6, therefore its nucleus is composed of 6 protons, 6 neutrons and has 6 electrons in its orbitals, which are distributed two by two between the 1s, 2s and 2p levels. Carbon Electron configurationĬarbon belongs to period 2 and group 14 in the periodic table. The most common use given to this element is for the manufacture of hydrocarbons ( Hydrogen + Carbon) and fossil-based fuels which are renewable energy enhancers. This element can be found as diamond, graphite etc. The reason why this element is so important is found in the physical and chemical properties of carbon. Carbon Atomic Number & Atomic MassĬarbon (symbol- C) is a nonmetal with atomic number 6 & atomic mass 12.0107 u, which is located in the second period and in group 14 of the periodic table.Ĭaron allows the formation of three isotopes naturally, carbon 14, 13 and 12. It is present in nature as carbon and diamonds, forming inorganic compounds such as CO 2 or organic compounds such as oil.

Its existence has been known since ancient times and is the basis of organic chemistry. It is the fourth most abundant element in mass in the universe and the 15th on Earth and the second in the human body.Ĭarbon, with symbol C, is a non-metallic and tetravalent chemical element, which means that it has four electrons to form chemical bonds. This is possible because carbon is capable of forming an enormous variety of stable chemical compounds, which is why it is considered by many to be the king of chemical elements.Ĭarbon is a chemical element, represented by the symbol C. The word "carbon" comes from the Latin "carbo" which designated coal and from the Roman "adamas" which means "hard steel". The entire science of organic chemistry, which is the study of carbon compounds, is based on carbon.Carbon is one of the most important elements of the periodic table for living beings since it forms the basis of life as we know it. No form of life on the planet earth is without carbon in its structure. Every plant, animal, and everything in between that is alive is based on carbon. The most important impact carbon has on the human race is the fact that carbon is the basic building block of life, as we know it. As an example, solid carbon is used to reduce iron from its oxide (Fe+3 to Fe0) in the blast furnace or other similar processes in which metallic elements are reduced from their oxide ores. Its ability to combine with oxygen makes it both a powerful and useful reducing agent (a substance that donates electrons resulting in reduction of the charge of the ion or atom being reduced). Carbon has a particularly strong affinity for oxygen either in the form of gaseous oxygen, or as oxygen contained in chemical combination with other elements. Pure carbon is a relatively reactive element and combines directly with many chemical elements, especially those considered oxidizing agents. Depending on the amount of graphite, amorphous carbon, or other contaminating elements, diamond can be found in colors ranging from clear water white through shades of black, gray, yellow, red, orange, blue, and green. Most forms of carbon, excluding diamond, are black to grey-black in color. Some of these forms include hexagonal graphite, rhombohedral graphite, diamond, buckminsterfullerene, and amorphous carbon (not really a crystalline form). There are a number of forms of carbon, known as allotropes, which are composed of pure carbon atoms but are arranged in different crystal lattices.

#Carbon electron configuration art free
Carbon is one of the few elements that occur in nature in its native or free elemental form.


 0 kommentar(er)
0 kommentar(er)
